Volumes & Issues
Contact
For any inquiries regarding journal development, the peer review process, copyright matters, or other general questions, please contact the editorial office.
Editorial Office
E-Mail: biofunmat@elspub.com
For production or technical issues, please contact the production team.
Production Team
E-Mail: production@elspub.com
No items found.
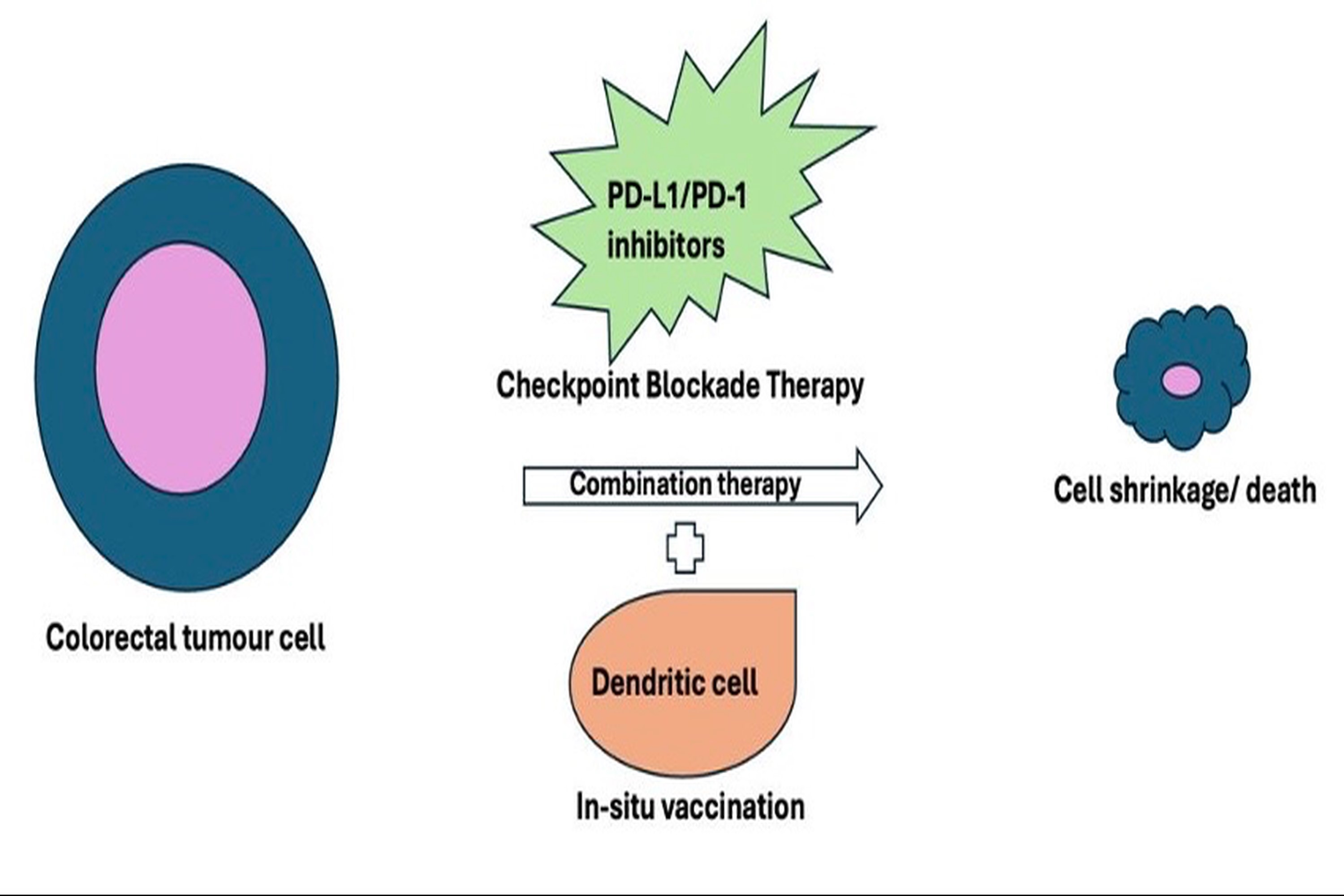
Integrating in situ vaccination with checkpoint blockade therapy: a novel approach to enhance anti-tumour immunity in colorectal cancer
DOI: 10.55092/bm20250007
Received: 15 Mar, 2025
Accepted: 29 Apr, 2025
Published: 06 May, 2025
Colorectal cancer (CRC) remains a challenging disease due to its high diversity and complex immune escape mechanisms. The anticipated incidence of colorectal cancer is expected to reach 3,200,000 new cases per year, indicating a 63% increase, with an estimated 1,600,000 deaths occurring annually, which signifies a 73% rise by the year 2040. In contrast to current treatment modalities, such as chemotherapy, radiotherapy, and surgical interventions, immunotherapy has significantly enhanced both survival rates and overall well-being for patients diagnosed with CRC. One of the new immunotherapeutic options that shows promise in colorectal cancer treatment is immune checkpoint inhibitors (ICIs). By removing the immune system's inhibition, ICIs allow functioning cytotoxic T cells to identify and eradicate cancerous cells. The primary issue with checkpoint inhibition is the rise in autoimmune adverse events that limit treatment. In situ vaccination (ISV) also offers a promising strategy to enhance anti-tumour immunity by delivering tumour-specific antigens directly to the tumour microenvironment. In situ vaccination, facilitated by dendritic cells, promotes robust T-cell priming and memory formation within the tumour microenvironment. This review discusses the prospects of combining ISV with checkpoint inhibitors, which can enhance immune cell function. This dual approach may lead to a more potent and durable anti-tumour effect with minimal adverse events, ultimately contributing to significant improvements in tumour regression, overall survival, and the overall well-being of individuals diagnosed with CRC.
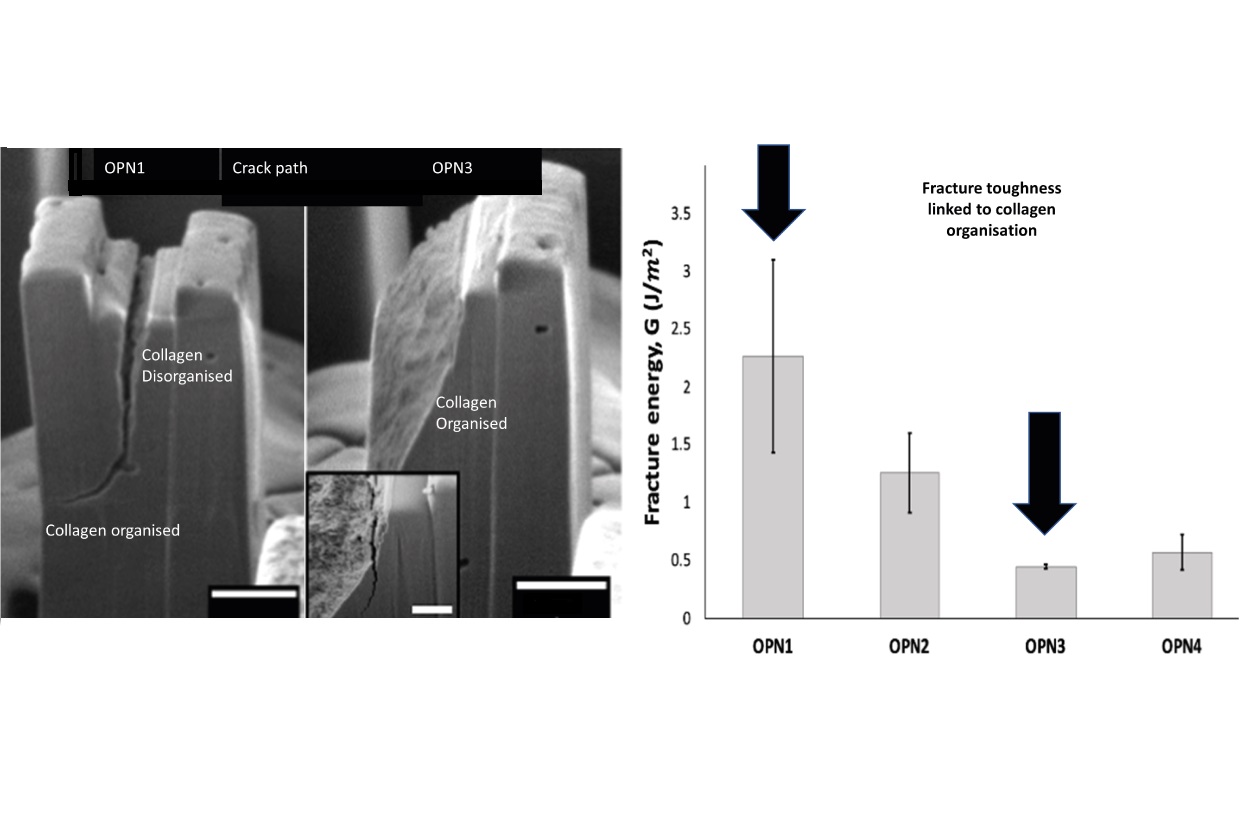
Micromechanics of osteopontin-deficient bone
DOI: 10.55092/bm20240005
Received: 01 May, 2024
Accepted: 21 Jun, 2024
Published: 01 Jul, 2024
Ageing- or bone-related diseases, such as osteoporosis leads to perturbations in the collagenous framework and mineralization that translate to deteriorated fracture resistance at the whole-bone level. However, bulk mechanical testing is insufficient to isolate the effect of these alterations on the mechanical response at a smaller length scale where molecular modifications manifest. Here, we combine in situ micromechanical testing using micropillars to determine elastic moduli, double cantilever beam mechanical tests to measure fracture toughness, and transmission electron microscopy (TEM) relate crack propagation at the microscale to local variations in collagen fibril organization. An osteopontin (OPN) knock out bone model with nanometer scale with regions of organised and disorganised collagen matrix and deteriorated fracture resistance at the whole-bone level was used to explore whether it is possible to propagate a crack in a transversely orientated pillar if the collagen fibrils in the pillar are disorganized. The average measured fracture energy for OPN-deficient mouse bone at this length scale, in the transverse direction was 0.94 ± 0.67 J/m2. This value is significantly lower than wild type bone, which we found in previous studies to be approximately 20 J/m2. TEM of cross-sections of the cracked pillars showed that the lack of OPN caused disorganization of the fibrillar network, possibly leading to deteriorated fracture resistance in bones. These preliminary findings indicate that OPN may contribute to bone’s fracture resistance through collagen matrix organization. This study serves as a starting point for more in-depth investigations that use in situ micromechanical testing using micropillars to study interplay between the ultrastructure and fracture resistance in pathologic bone.
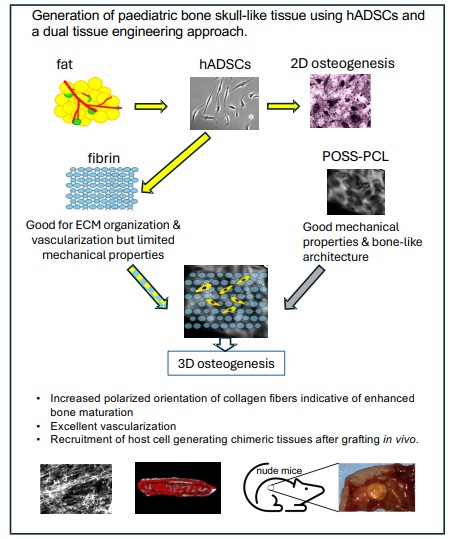
Mimicking 3D bone microenvironment using a hybrid hydrogel-nanocomposite scaffold and human adipose-derived stem cells for bone differentiation and vascularization
DOI: 10.55092/bm20240002
Received: 12 Dec, 2023
Accepted: 18 Mar, 2024
Published: 12 Apr, 2024
Bioengineered bone tissues could bypass limitations of traditional reconstructive methods such as bone grafts or foreign-body implants, but to date this approach has been limited by immaturity of engineered bones and vascularization. Pediatric human adipose-derived stem cells (hADSCs) can be derived using minimally invasive procedures and are known to undergo osteogenic differentiation. Here we have taken a “design-in reverse” approach to tissue engineering by mimicking the native tissue microenvironment to bioengineer complex vascularized tissues. Specifically, we sought to optimize bone tissue engineering by modelling the bone tissue microenvironment and vascularization using hADSCs in combination with a nanocomposite biodegradable nanomaterial and hydrogel, POSS-PCL-fibrin. Our scaffolds exhibited similar architectural features to bone and supported hADSC osteogenic differentiation in vitro. Extensive vascularization and formation of tissues resembling pediatric calvarial (upper part of the skull encasing the brain) bone as well as host cell infiltration yielding chimeric tissues were achieved in vivo in nude mice. Our results suggest a promising dual tissue engineering purpose for POSS-PCL-fibrin both as a scaffold for bioengineering prefabricated replacement bone tissues and potentially as a bioactive scaffold for in situ bone regeneration.
No items found.
No items found.
No items found.